Smelting Process of Steel and its Chemical Reaction Equation
Steel-making begins with iron. Steel comes from iron. Pig iron produced by smelting with iron ore has a high carbon content (>2.08%) and contains many impurities (such as silicon, manganese, phosphorus, sulfur, etc.). As a result, pig iron lacks plasticity and toughness, and its mechanical properties are poor. Besides melt casting, it can not be processed under pressure, which limits its use.
In order to overcome these shortcomings of pig iron and make it play a more important role in the industry, it is also necessary to use various sources of oxygen at high temperatures to remove impurities to a certain extent in order to obtain a certain composition and quality of iron-carbon alloy - steel. This method of removing impurities from pig iron by oxidation at high temperatures is called steelmaking.
Basic Principles of Steelmaking
Various impurities in pig iron have a strong affinity with oxygen to varying degrees at high temperatures. They can therefore be oxidized into liquid, solid, or gas oxides, which act at high temperatures with the lining and the flux added into the furnace, combine to form slag, and are removed from the furnace when slag is scraped, and CO is carried out when the molten steel boils.
In the steel-making furnace, the oxidation of impurities is mainly realized by the presence of FeO.
2Fe+O2→2FeO
2Fe+O2_2FeO
Oxidation of Silicon
Silicon has a strong affinity to oxygen, so it oxidizes very quickly and is completely oxidized at the beginning of smelting to produce SiO2:
>>Si+2FeO→SiO2+2Fe
>Si+2FeO_SiO2+2Fe
At the same time, SiO2 reacts with FeO to form silicates:
>>2FeO+SiO2→2FeO·SiO2
>2FeO+SiO2_2FeO.SiO2
This salt is an important part of slag. It reacts with CaO to form stable compounds 2CaO SiO2 and FeO, which are firmly present in the slag, while the latter becomes free in the slag and increases the content of FeO in the slag. It is advantageous to promote the oxidation of impurities. The reaction is as follows:
>>2FeO·SiO2+2CaO→2CaO·SiO2+2FeO
>2FeO.SiO2+2CaO_2CaO.SiO2+2FeO
Oxidation of Manganese
Manganese is also an oxidizable element. The MnO produced by it has a higher melting point. MnO is not soluble in metal solution, but it floats on the liquid metal surface with SiO2 forming compounds and forms part of the slag.
>>Mn+FeO→MnO+Fe
>Mn+FeO_MnO+Fe
>>2MnO+SiO2→2MnO·SiO2
>2MnO+SiO2_2MnO.SiO2
The oxidation of silicon and manganese emits a great deal of heat, which can rapidly increase the furnace temperature (which is particularly important for converter steelmaking) and greatly accelerate the oxidation of carbon.
Oxidation of Carbon
Carbon oxidation takes up a lot of heat energy and must be carried out at higher temperatures. The oxidation of carbon is also an important reaction in steel-making:
>>C+FeO→CO+Fe
>C+FeO_CO+Fe
Because CO gas is produced when carbon is oxidized, it has a strong stirring effect when escaping from liquid metals, which is called boiling. The results of boiling can promote the uniformity of composition and temperature of the molten pool, accelerate the reaction between metal and slag, and also facilitate the removal of gas and inclusions in the steel.
Oxidation of Phosphorus Element
Oxidation of phosphorus can take place at relatively low temperatures. The dephosphorization process is a combination of several reactions, the following reactions:
>>2P+5FeO→P2O5+5Fe
>2P+5FeO_P2O5+5Fe
>>P2O5+3FeO→3FeO·P2O5
>P2O5+3FeO_3FeO.P2O5
The following reactions occur when sufficient CaO is present in the alkaline slag:
>>3FeO·P2O5+4CaO→4CaO·P2O5+3FeO
>3FeO.P2O5+4CaO_4CaO.P2O5+3FeO
The resulting 4CaO.P2O5 is a stable compound that remains firmly in the slag and thus achieves the purpose of dephosphorization.
It must be noticed that iron silicate and ferromanganese etc. are added to the deoxidizing process of molten steel. As a result, slag often becomes acidic after deoxidizing, which destroys 3FeO.P2O5, from which P2O5 is reduced, while P2O5 is an unstable oxide, which is easily reduced by carbon and results in phosphorus return at high temperature. This also shows that it is very difficult to remove phosphorus in acid furnaces. In order to prevent this phenomenon, it is necessary to properly increase the alkalinity and quantity of slag and improve the oxidation of slag.
Oxidation of Sulfur
Sulfur is present in the form of FeS. Sulfur can also be removed when there is enough CaO in the slag as follows:
>>FeS+CaO→CaS+FeO
>FeS+CaO_CaS+FeO
The resulting CaS is not soluble in the molten steel, but slag floats on the surface of the molten steel.
The above reaction is reversible and takes place in the slag containing FeO. When FeO reacts with CaS, sulfur will return to the molten steel, so the desulfurization efficiency will increase with the decrease of FeO content in the slag. When there is enough carbon in the residue, the reaction is different:
>>CaO+FeS+C→CaS+Fe+CO
>CaO+FeS+C_CaS+Fe+CO
Because carbon robs oxygen from FeO and loses the possibility of interaction between CaS and FeO, the reaction can not be reversed, which is why desulfurization in electric furnace steelmaking is more complete than in the other two methods.
Manganese also plays a role in promoting desulfurization during the desulfurization process as follows:
>>FeS+MnO→MnS+FeO
>FeS+MnO_MnS+FeO
The resulting MnS is almost insoluble in molten steel and enters the slag. Therefore, desulfurization increases with the oxidation of manganese.
Deoxygenation of FeO
After the above series of oxidation reactions, although the impurities have been oxidized to achieve the purpose of removal, also because of the oxidation result, there is more FeO in the steel water, which means that there is a lot of oxygen in the steel water, bringing great harm to the steel, on the one hand, steel with a lot of air bubbles; On the other hand, it also causes hot and cold brittleness of steel, and the hazard increases with the increase of carbon content.
Therefore, at the end of the steelmaking process, it is also necessary to try to remove a lot of oxygen from the molten steel. The usual method is to add some deoxidizers such as ferromanganese, ferrosilicon, and aluminum to the molten steel. They strongly take oxygen from FeO to achieve the purpose of deoxidation. The reaction is as follows:
FeO+Mn→MnO+Fe
FeO+Mn_MnO+Fe
2FeO+Si→SiO2+2Fe
2FeO+Si_SiO2+2Fe
3FeO+2Al→Al2O3+3Fe
3FeO+2Al_Al2O3+3Fe
Effect of Slag
The whole steelmaking process consists of oxidation and reduction processes. Oxygenation of carbon, silicon, manganese, and phosphorus is usually called a reaction in the oxidation period and desulfurization and deoxidation are called reactions in the reduction period. As can be seen from the above reaction formulas, many factors must be considered in order to remove impurities from metals, but the most important ones are slagging and slag removal.
Slag Plays the Following Important Roles in Steelmaking:
(1) Slag shall ensure that the steelmaking process is carried out in a certain reaction direction (oxidation or reduction).
(2) Slag shall ensure the maximum removal of harmful impurities (phosphorus and sulfur) from metal and the prevention of gas (nitrogen and hydrogen) from furnace gas entering metal.
(3) Slag shall ensure minimal loss of iron and other valuable elements during operation.
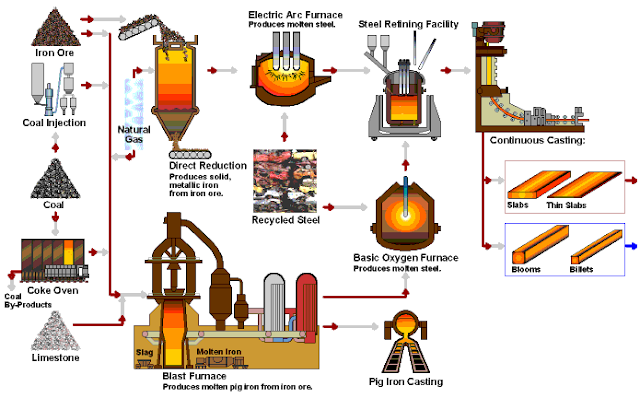
Basic Methods of Steelmaking
1. Converter Steelmaking
Converter steelmaking is a steelmaking method that uses air or oxygen to oxidize elements in molten iron to specified limits by bottom, side, and top blowing, so as to obtain qualified steels.
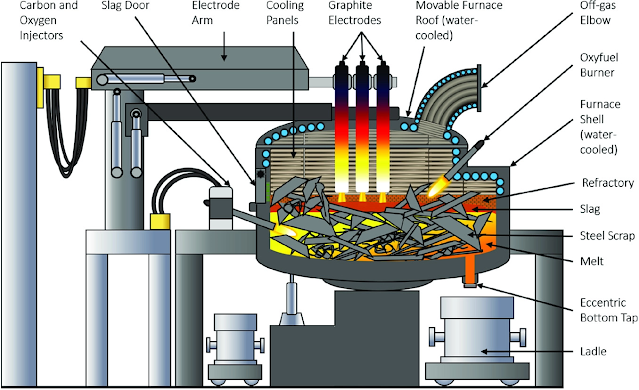
2. Electric Furnace Steelmaking
Electric furnaces are steelmaking by converting electrical energy into thermal energy. There are two types of electric furnaces commonly used: electric arc furnaces and induction furnaces. The electric arc furnace is widely used and suitable for smelting high-quality steel and alloy steel. Induction furnaces are used for smelting high-grade alloy steels and non-ferrous alloys.
3. Open-hearth Steelmaking
With the development of industry, a large amount of scrap steel has accumulated in the metal processing industry. At that time it could not be converted back into steel, so steelmaking workers were looking for a method of making steel from scrap steel. Open-hearth steelmaking was invented by Martin in France in 1864.
The rapid development of oxygen top-blown converter steelmaking will replace open-hearth steelmaking. Oxygen top-blown converter and electric furnace are the main new steelmaking workshops in China.
With the progress of science and technology, new steel-making methods, such as vacuum treatment of molten steel, electro slag furnace smelting, and vacuum induction furnace smelting, have been used more and more.
The Casting of Steel Ingots
The molten steel obtained from the steelmaking furnace must be shaped into ingots for subsequent processing (rolling and pressing). This kind of ingot, which is in the middle of the molten steel and the steel from the factory, is called the ingot. The ingot is solidified by pouring molten steel (molten steel) into the mold through a ladle (also called a ladle).
The process of ingot casting by casting is abbreviated as ingot or die casting. The process of billet casting by continuous casting steel method is abbreviated as continuous casting.
Die Casting Process
First transfer the molten steel in the steelmaking furnace into the ladle, then lift the ladle over the ingot mold, and then pour the molten steel into one or more ingot molds intermittently. After the molten steel solidifies, the ingot is demoulded. After demolding, the ingot is cut to the end and sent to the heating furnace for heating. At last, the steel embryo is obtained by one or more initial rolling embryos.
The steel ingots used for bar and profile production are usually square sections (called square ingots). The steel ingots for plate production are generally rectangular sections (called flat ingots). The steel ingots for forging and pressing are square, round, and polygonal.
The ingot casting process is accompanied by various physical and chemical phenomena, such as heat conduction, volume shrinkage, liquid steel flow, carbon and oxygen reaction, component segregation, etc. This results in different crystalline structures and component distributions.
In the process of ingot casting, various defects will occur due to improper operation, improper injection speed, and improper temperature control. Common defects are ingot surface scarring, heavy skin, longitudinal and transverse cracks, internal residual shrinkage holes, subcutaneous bubbles, looseness and segregation, inclusions caused by refractories and slag, and dust mixed in steel, etc. These defects can greatly reduce the billet yield of the ingot and even scrap the entire ingot.
Free send inquiries to stella@hanrm.com if any needs.
Email: stella@hanrm.com Or stellarollingmill@gmail.com
Whatsapp/Wechat:+8615877652925
Website: https://www.hanrm.com


.jpg)








没有评论:
发表评论